Haplothrips (Haplothrips) gowdeyi (Franklin, 1908)
Phlaeothripinae, Phlaeothripidae, Tubulifera, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, pedicel and segments III-V (segments III and IV with simple sense cones)
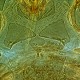
Fig. 2: Head dorsal with ocellar triangle and bridge between maxillary stylets
Fig. 3: Pronotum
Fig. 4: Prosternal plates
Fig. 5: Meso- and metanotum
Fig. 6: Fore wing and fore wing distal region
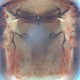
Fig. 7: Tergites I (pelta) and II
Fig. 8: Tergites IV and V with wing-retaining setae
Introduction and recognition
Haplothrips (Haplothrips) gowdeyi is found commonly in the flowers of a wide range of plants, mainly on Poaceae, often on Fabaceae. Female macropterous; body and legs dark brown (Fig. 6); fore tarsi light brown; antennal segments III & IV yellow, V-VI yellow in basal half but light brown at apex (Fig. 1); fore wings pale with extreme base shaded (Fig. 6); major setae dark brown except on tergite IX. Antennae 8-segmented; segment III with 2 sense cones, IV with 4 sense cones. Head slightly longer than wide; postocular setae weakly capitate and about as long as width of compound eye, arising close to posterior margin of eye; maxillary stylets retracted to postocular setae, about one third of head width apart with distinct maxillary bridge (Fig. 2). Pronotum with 5 pairs of slender, capitate major setae (Fig. 3); epimeral suture complete. Prosternal basantra well developed and broader than long; mesopraesternum complete and boat-shaped but very narrow medially (Fig. 4); metathoracic sternopleural sutures not developed. Fore tarsus with a small tooth near apex. Fore wing constricted medially; with about 8 duplicated cilia (Fig. 6). Pelta triangular (Fig. 7); tergites II-VII each with 2 pairs of sigmoid wing-retaining setae (Fig. 8); tergite IX setae finely acute and as long as tube; tube shorter than head width; anal setae no more than 1.5 times as long as tube.
Male macropterous; smaller than female; fore tarsus with tooth variable in size; tergite IX setae S2 short and stout; sternite VIII without glandular area.
Taxonomic identity
Species
Haplothrips (Haplothrips) gowdeyi (Franklin, 1908)
Taxonomic history
Haplothrips mahensis Bagnall, 1921
Anthothrips dozieri Watson, 1918
Haplothrips karnyi Bagnall, 1913
Haplothrips brevicollis Bagnall, 1913
Haplothrips soror Schmutz, 1913
Haplothrips sororcula Schmutz, 1913
Anthothrips variabilis Crawford, 1910
Anthothrips usitatus Bagnall, 1910
Anthothrips gowdeyi Franklin, 1908
Common name
-
Present taxonomic position
Family: Phlaeothripidae Uzel, 1895
Subfamily: Phlaeothripinae (Uzel) Priesner, 1928
Genus: Haplothrips Amyot & Serville, 1843
Subgenus: Haplothrips (Haplothrips) Amyot & Serville, 1843
Genus description
The genus Haplothrips (Haplothrips) Amyot & Serville, 1843
With nearly 250 described species, Haplothrips is the third largest genus of Thysanoptera. Most of these species, largely from Europe and the Old World tropics, are flower-living, including the flowers of grasses and sedges. Members of the genus are mostly macropterous and have duplicated fringe cilia on the fore wing (subgenus Haplothrips), with the fore wing constricted medially and contain well developed paired prosternal basantra (Mound & Kibby 1998). The species in this group which contain single fringed cilia on the fore wing belong to the subgenus Trybomiella. In addition, Haplothrips species have 4 sense cones on antennal segment IV, the head contains a well developed maxillary bridge, complete epimeral sutures on the pronotum, a triangular shaped pelta, and tergites II through VII exhibit 2 pairs of wing-retaining setae.
Species description
Typical key character states of Haplothrips (Haplothrips) gowdeyi
Coloration
Body color: mainly brown to dark brown
Antennae
Number of antennal segments: 8
Segment III - number of sense cones: 2
Segment IV - number of sense cones: 4
Form of sensorium on antennal segments III and IV: emergent and simple on segments III and IV
Head
Maxillary stylet position: about one third of head width apart
Maxillary bridge: present
Postocular setae: about half to nine tenths as long as compound eye
Cheeks: without seta-bearing tubercle
Prothorax
Number of pairs of elongate pronotal setae: 5
Prosternal basantra: present, wider than long
Epimeral suture: complete
Mesothorax
Shape of mesopraesternum: complete and boat shaped, but very narrow medially
Wings
Fore- and hind wings: present and more than half as long as abdomen
Fore wing veins: absent
Fringe cilia arising: not from sockets
Fore wing shape: constricted medially
Fore wing duplicated cilia: present
Fore wing color: uniformly pale or weakly shaded
Fore wing extreme apex color: pale
Legs
Fore tarsus: with a tooth near apex
Abdomen
Abdominal tergites III-V: with 2 pairs of sigmoid curved wing-retaining setae
Abdominal segment 10: complete tube in both sexes
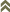
Similar or related species
Compared to Haplothrips clarisetis (subgenus Trybomiella) with only single fringed cilia on the fore wing, Haplothrips gowdeyi (subgenus Haplothrips) has duplicated fringe cilia on the fore wing. Other distinguishing features are: 5 pairs of elongate pronotal setae (Haplothrips clarisetis with only 4 pairs of these setae because the pronotal midlateral setae are no larger than discal setae), mesopraesternum complete and boat-shaped, but very narrow medially (in Haplothrips clarisetis mesopraesternum reduced to two lateral triangles), and fore tarsus in females with a small tooth (Haplothrips clarisetis without a tooth in females).
Karnyothrips flavipes is similar to Haplothrips species in structure, but has a prosternal basantra that is about as broad as long or longer than wide (Haplothrips with a basantra that is distinctly broader than long), anal setae 1.5 to 2.0 times as long as tube or longer (Haplothrips with anal setae no more than 1.5 times as long as tube), anteromarginal pronotal setae not longer than discal setae (Haplothrips with anteromarginal setae usually longer than discal setae), and a fore tarsal tooth near the apex that is forwardly directed and hook-like (Haplothrips without or with a small lateral tooth).
Almost all species of of Phlaeothripidae have a mainly brown body color, uniformly fore wings and tergites II-VII each with 2 pairs of wing-retaining setae (only Aleurodothrips fasciapennis with a bicolored body, fore wings with transverse alternating bands of dark and light, and tergites II-VII each with 1 pair of wing-retaining setae), and no seta-bearing tubercle on cheeks (only Hoplandrothrips marshalli has cheeks with at least 1 seta-bearing tubercle in basal third or 3 pairs laterally). Haplothrips species as well as Karnyothrips flavipes and species of Gynaikothrips have a maxillary stylet is about one third of head width apart, and the length of postocular setae is about half to nine tenths as long as compound eye (in Aleurodothrips fasciapennis the maxillary stylet is more than half of head width apart, and postocular setae are shorter than distance of the setal base from the eye; in Hoplandrothrips marshalli the maxillary stylet is about one fifth of head width apart, and postocular setae are as long as dorsal length of compound eye or longer). Species of Haplothrips and Karnyothrips flavipes possess a maxillary bridge and prosternal basantra (species of Gynaikothrips, Hoplandrothrips marshalli and Aleurodothrips fasciapennis have neither a maxillary bridge nor a prosternal basantra). Haplothrips (Haplothrips) gowdeyi as well as Karnyothrips flavipes and Hoplandrothrips marshalli, and species of Gynaikothrips have duplicated cilia on posterior margin of fore wing (Aleurodothrips fasciapennis and Haplothrips (Trybomiella) clarisetis without duplicated cilia on posterior margin of fore wing).
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The full cycle can take less than one week to over a month and adults may live for more than one month producing several generations in one year depending on seasonal weather (Lewis 1973).
Host plants
Mainly on Poaceae, often on Fabaceae.
Crops: amaranth, chillies, citrus, cucumber, date palm (Phoenix dactylifera), French bean, grape vine, groundnut, ice plant (Carpobrotus edulis), maize, onion, potato, pumpkin, redgram, ridge gourd (Luffa), rize, sunflower, sweet potato, tomato.
Weeds: Achyranthes aspera, Asystasia mysorensis, Bidens pilosa, Canna indica, Clitoria sp., Cynoglossum geometricum, Erucastrum arabicum, Flaveria sp., Galinsoga parviflora, Gamolepis chrysanthemoides, Gliricidia sp., Heliotropium zeylanicum, Lactuca inermis, Lantana camara, Leonotis nepetifolia, Nicandra physalodes, Ocimum sp., Osteospermum sp., Polygonum pulchrum, Senecio diversifolius, Solanum incanum, Tagetes minuta, Tithonia diversifolia.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
-
Detection and control strategies
-
Additional notes
Males are rarely collected.
Biogeography
Judging from the infrequency of males in collections from countries other than some African territories, this species is probably African in origin. However, it is now widespread throughout the tropics and subtropics. Cape Verde Islands (Santo Antão; Sao Nicolau; Sao Tiago; Fogo),
Egypt (Alexandria),
Ivory Coast,
Kenya (Kibos),
Nigeria,
Seychelles,
South Africa (in all parts),
Sainte-Hélèn,
Tanzania (Kibosho, Arusha),
Tunisia (Skanés), Uganda.
African countries where Haplothrips (Haplothrips) gowdeyi has been reported
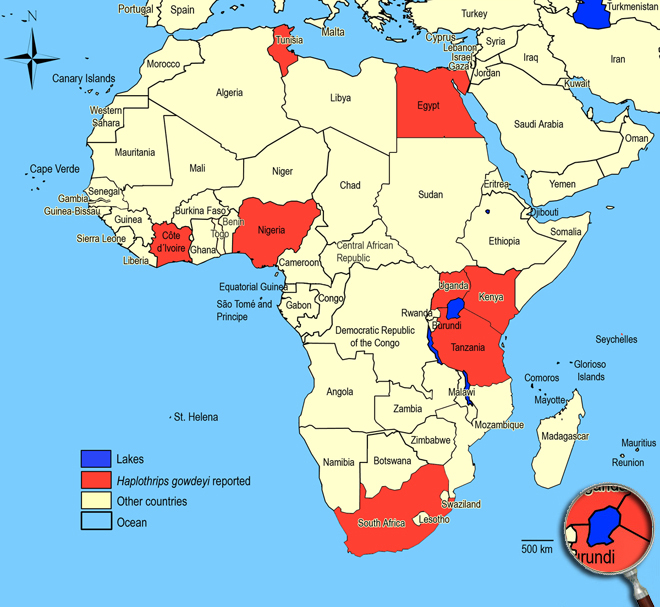
Occurence of Haplothrips (Haplothrips) gowdeyi in East Africa
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Haplothrips (Haplothrips) gowdeyi in parts of East Africa.
Bibliography
Bagnall RS (1910). Thysanoptera, pp. 669-701. In Sharp D [ed.], Fauna Hawaiiensis being the land-fauna of the Hawaiian Islands, Vol. 3, Part 6. Cambridge University Press, London
Bagnall RS (1913). Brief descriptions of new Thysanoptera - I. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 8) 12: 290-299
Bagnall RS (1921). On Thysanoptera from the Seychelles Islands and Rodrigues. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 7: 257-293
Childers CC & Nakahara S (2006). Thysanoptera (thrips) within citrus orchards in Florida: Species distribution, relative and seasonal abundance within trees, and species on vines and ground cover plants. Journal of Insect Science. 6 (45): 1-19
Crawford DL (1910). Thysanoptera from Mexico and the South II. Pomona College Journal of Entomology. 2 (1): 153-170
Franklin HJ (1908). On a collection of thysanopterous insects from Barbados and St. Vincent Islands. Proceedings of the United States National Museum. 33 (1590): 715-730
Jenser G (1982). Data to the Thysanoptera fauna of Tunisia. Folia Entomologica Hungarica. 43 (1): 55-57
Karny H (1912). Revision der von Serville aufgestellten Thysanopteren-Genera. Zoologische Annalen. 4 (4): 322-344
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp Wissenschaften, Hohenwarsleben, 384 pp. ISBN 13: 978 3 89432 8917
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Mound LA (1968). A review of R. S. Bagnalľs Thysanoptera collections. Bulletin of the British Museum (Natural History), Entomology. Supplement 11: 1-181
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR (1973). A revision of the Australian Haplothripini, with descriptions of three new species (Thysanoptera: Phlaeothripidae). Journal of the Australian Entomological Society. 12: 315-339
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Priesner H (1929). Contribution towards a knowledge of the Thysanoptera of Egypt, II. Bulletin de la Société Royale Entomologique ďEgypte. 13 (4): 211-219
Priesner H (1931). A review of the African Haplothrips-species (Thysanoptera). Bulletin de la Société Royale Entomologique ďEgypte. 14 (4): 230-277
Schmutz K (1913). Zur Kenntnis der Thysanopterenfauna von Ceylon. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Abteilung I. Biologie, Mineralogie, Erdkunde. 122 (7): 991-1089
Watson JR (1918). Thysanoptera of Florida. Florida Buggist. 2: 65-77
zur Strassen R (1958). Notes on Thysanoptera of tropical Africa and St. Helena. Journal of the Entomological Society of Southern Africa. 21 (1): 69-79
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern. Africa 23 (2): 321-367
zur Strassen R (1976). La fauna terrestre de ľile de Sainte-Hélène III - 18. Thysanoptera. Annales de la Musee Royal de ľAfrique Central, Serie 8, Sciences Zoologiques. 215: 236-256
zur Strassen R (1992). Phlaeothripidae von den Kapverdischen Inseln (Insecta: Thysanoptera). Senckenbergiana Biologica. 72 (1-3): 139-171
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California